Surgical Face Mask
Product Lifecycle Quality Management
Testing, Inspection, Audit and Certification for Face Masks
Face Mask Product Lifecycle Quality Management
Download Service SheetBUSINESS CHALLENGE
Manufacturers worldwide are transforming production lines to meet the growing needs of people worldwide for surgical masks. As a buyer or seller, how confident are you in your process and product controls in meeting regulatory as well as brand specifications in these most challenging times.
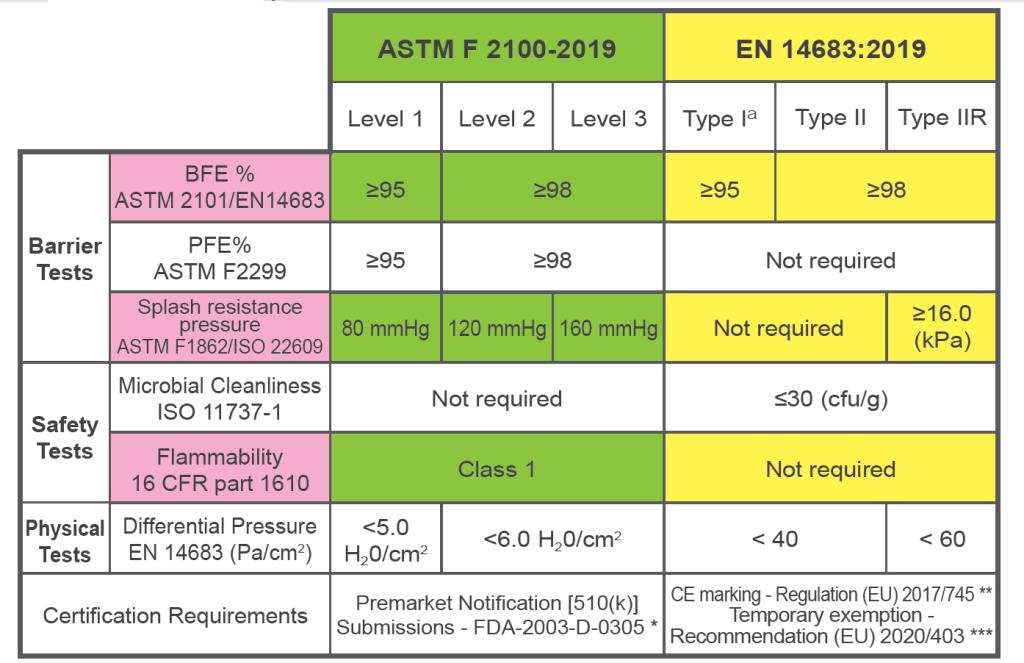
For two of the largest markets, the testing and certification requirements for USA (ASTM) and Europe (EN) are detailed in the table below:
* Premarket Notification [510(k)] Submissions - FDA-2003-D-0305
** CE marking - Regulation (EU) 2017/745
*** Temporary CE marking exemption - Recommendation (EU) 2020/403
Temporary CE marking Exemption
RECOMMENDATION (EU) 2020/403 of 13 March 2020 was released within the context of the COVID-19 threat to address the shortage of surgical face masks.
Where non-CE marked surgical face masks are intended to enter the EU market, the relevant market surveillance authorities will evaluate the products and, if found to be compliant with the essential health and safety requirements, will take measures to allow the placing on the Union market for a limited period of time, or while the conformity assessment procedure with the notified body is being carried out.
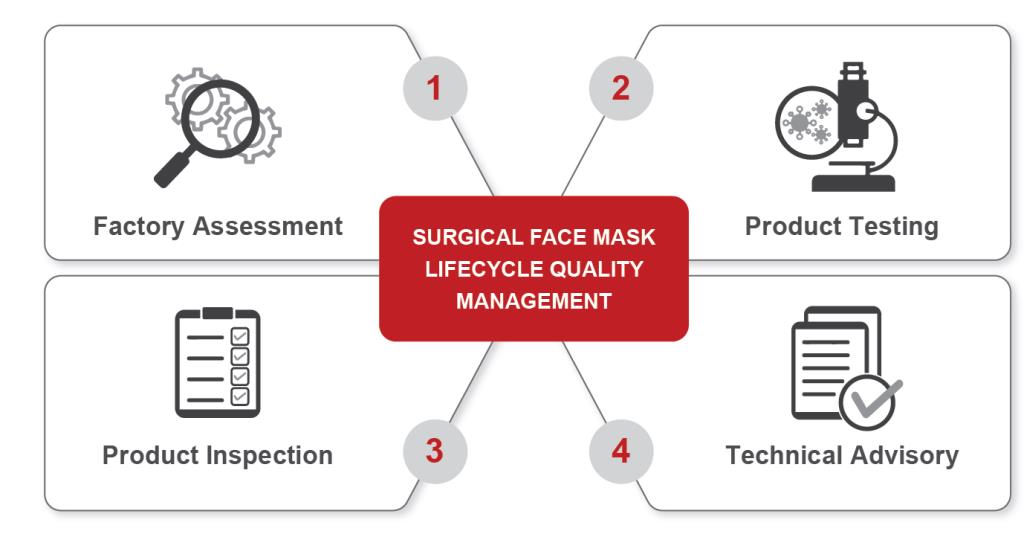
BUREAU VERITAS SOLUTIONS
Factory Assessment: Supports with assessment of conformity with the special arrangements given to address the COVID-19 outbreak. Specific requirements for surgical face masks include:
- sterilization facilities;
- disinfection areas;
- clean rooms;
- proper protective clothing
- storage;
- …
Product Testing: Microbiological hub in Sri Lanka provides Bacterial Filtration Efficiency (BFE) and associated testing for surgical face masks against EN 14683 and its counterpart committee requirements in the U.S. (ASTM F2101).
Product Inspection: Bureau Veritas has trained inspectors in major face mask production countries worldwide. The inspection protocol addresses quantity, labeling and specification review as well as a variety of on-site testing including:
- product dimension measurement;
- nose clip reliability;
- tensile test to the mask connection points;
- claimed function;
- actual wear test;
- ...
Technical Advisory:
- Level 1: Document Review: Adequacy check of documentation including the applicable test requirements, third party lab accreditation, vendor information and overall rating;
- Level 2: CE marking Access. Our specialists can help you achieve formal CE marking from an appointed Notified Body or surveillance authority exemption acceptance.
FAQ - Face Mask Compliance & Quality Control
Download FAQ